Siderophores: A treatment target?
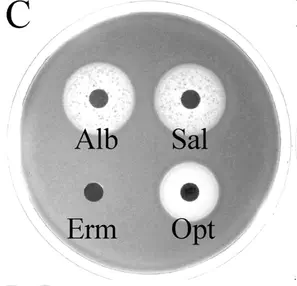 Strain of Streptococcus pneumoniae sp. that is sensitive to the sideromycins albomycin and salmycin but not the antibiotic erythromycin. Source, Fig 4C.
Strain of Streptococcus pneumoniae sp. that is sensitive to the sideromycins albomycin and salmycin but not the antibiotic erythromycin. Source, Fig 4C.
In previous Microbial Science blog posts, I described how many bacterial pathogens rely on siderophores to help scavenge iron during infections as well as protect them from the immune response or competing bacterial species. But is there a way to take what we’ve learned about these essential molecules and use them to turn the tide against the wave of antibacterial resistance? Researchers are developing sideromycins, inhibitors and vaccines to turn siderophores against their makers.
Sideromycins
To get an edge in survival against competing bacterial species, some bacteria (e.g. Streptomyces spp.) make sideromycins, siderophores conjugated with an antibiotic molecule. Sideromycins look like siderophores to other species of bacteria, which import them for their iron payload. But by bringing sideromycins in, the bacteria inadvertently poison themselves with the attached antibiotic. Researchers have caught onto this trick and designed their own sideromycins to target bacterial pathogens. This is important work since antibacterial resistance is at an all-time high and the number of effective antibiotics is decreasing. Since most resistance mechanisms rely on keeping the antibiotics out, the Trojan Horse sideromycins might help antibiotics bypass these and target specific pathogens.
This is what researchers at the University of Notre Dame had in mind when they designed a sideromycin against the multidrug-resistant Acinetobacter baumannii. The authors conjugated the antibiotic daptomycin to the A. baumannii siderophore called fimsbactin and effectively treated infected mice. This is especially exciting because daptomycin is traditionally only effective against gram-positive bacteria, but by conjugating it to fimsbactin, they could use it to treat the gram-negative A. baumannii, but not others (e.g., E. coli, Pseudomonas). Other groups have generated sideromycins against Staphylococcus aureus, Providencia stuartii, Pseudomonas aeruginosa, and even A. baumannii, but this report is the first to show that the drugs can work in a mouse infection model. And unfortunately, it is possible for mechanisms to prevent sideromycin import to evolve, thus creating resistance against sideromycins.
Inhibitors
Another way to co-opt our knowledge of siderophores is to generate new antibiotics that target siderophores and prevent their production or use by pathogens. There are two common pathways for siderophore biosynthesis in bacteria, and researchers have taken advantage of these similarities to identify small molecules that interact with and inhibit siderophore biosynthesis enzymes. In some cases, researchers use rational design to generate these small molecules. By taking what they know about the structure of the enzymes and how they function, researchers can generate small molecules that they think will act as inhibitors. Two 2005 studies described how researchers targeted an early step in siderophore biosynthesis by Mycobacterium tuberculosis. One group designed molecules that interfered with a biosynthetic protein’s ability to bind ATP, while the other targeted the ligand binding site of a biosynthetic protein and was also able to inhibit siderophore biosynthesis by Yersinia pestis. More recently, Dr. David Sherman’s lab at the University of Michigan screened a library of naturally occurring small molecules for their ability to inhibit siderophore biosynthesis in Bacillus anthracis and Staphylococcus aureus. In all of these cases, the identified molecules inhibited growth of the pathogen in a test tube, but none have been tested in a mouse model of infection.
Vaccines
The best way to fight an infection is by preventing it from happening at all. With that in mind, Dr. Harry Mobley’s lab at the University of Michigan has been exploring siderophore-based vaccines against urinary tract infections. Initially, they tried to target the immune response against select siderophore receptors on the cell surface of urinary pathogenic E. coli (UPEC). With this method, they successfully vaccinated mice against the receptor for yersiniabactin and generate a protective immune response. But those proteins are unwieldy and difficult to purify for regular use in a vaccine. What about targeting the siderophore itself?
Generating protective vaccines that target siderophores has been considered a risky prospect since the molecules are so small and don’t seem to elicit immune responses. While a 2009 study demonstrated it was possible to generate antibodies against a siderophore (in this case vibriobactin), they didn’t show a protective immune response. But two papers published side-by-side in the Proceedings of the National Academy of Sciences last year showed how researchers have accomplished targeting the mouse immune response against siderophores. One study from the Mobley lab described their continued efforts to generate a vaccine against UPEC. Here, they conjugated the siderophores aerobactin and yersiniabactin to cationized bovine serum albumin—albumin protein modified to elicit a strong immune response—and successfully immunized mice against urinary tract infections. Unfortunately, Mobley’s group wasn’t able to demonstrate protective antibodies against the siderophores. According to the study’s first author Dr. Laura Mike, “it is possible that siderophores are stimulating the immune system in other ways; the small size of the siderophores are not as amenable to many of the standard immunological assays.” This makes it difficult to identify the type of immune response the mice were generating as a result of the vaccine.
But the second study, from Manuela Raffatellu’s lab at the University of California, Irvine, did detect antibodies against enterobactin, the siderophore of vaccination. Raffatellu’s group also conjugated the siderophore to an immunogenic protein but used cholera toxin subunit B because it helps elicit a strong mucosal response, which is important for protection against Salmonella. Raffatellu’s group was also successful in generating a vaccine that protected against the pathogen.
What’s especially promising, according to Dr. Mike, is that these studies have shown that it may not be necessary to conjugate the siderophore to the immunogenic protein, and that these vaccines had little effect on the mice microbiomes. So, in theory, researchers could “pick any virulence-associated siderophore for a vaccine antigen. We will still have to keep in mind that not all species of bacteria, or isolates within a single species, always use the same exact siderophores,” Mike says. But this offers a way to tailor the vaccine because “we can either create very narrow-spectrum vaccines by only using one or two siderophore antigens, or we could create a broader vaccine by incorporating more siderophores into our formulation,” she adds
Research into anti-siderophore treatment strategies appears promising. But none of them have been translated to an effective treatment has emerged from this work, and few of the putative therapy options have been tested in mice. This is an expensive step, but is necessary to identify which of these therapies have potential for clinical use.
*Republished from ASM.org with permission